Abstract
Background: Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) includes a history of myelodysplasia syndrome (MDS) and no MDS history (30% of AML, MDS-related cytogenetic abnormalities, and multilineage dysplasia) which was called MDS-like AML. We assume that traditional AML therapy combined with decitabine (DAC) may improve the effectiveness. We retrospectively analyzed 98 patients diagnosed as MDS-like AML and evaluated the curative effect and side effects of decitabine combined chemotherapy comparing to standard 3+7 induction protocol.
Patients and methods:This study analyzed AML patients aged 14-60 (exclude M3) diagnosed between 2014 to 2017. All patients were supposed to be on at least one of the conditions below: ① anemia, leucopenia, or thrombocytopenia for over 6 months; ② macrocytic anemia (MCV>95.0fL); ③ observation of marrow dyshaematopoiesis; ④immature cells >20% but with low percentage; ⑤FISH detected 5q-, 7q-, +8 or 20q-; ⑥methylation related gene mutation. Ninety-eight eligible patients were included (51 in DAC group; 47 in non-DAC group). 1. DAC group: 22 patients were treated with DAC+CAG, which included decitabine of 20 mg/m2 intravenously d1-5 and G-CSF of 300μg/day (d0-9) for priming combined with cytarabine of 20 mg/m2 for d3-9, aclarubicin of 7 mg/m2 d3-6. 29 patients received DAC+IA/MA regimen (IA for 25 cases, MA for 4 cases), included decitabine of 20 mg/m2 intravenously d1-5 combined with a standard "3+7" induction chemotherapy using standard dose of cytarabine (100 mg q12h) intravenously for day1-7 with idarubicin or mitoxantrone of 8 mg/m2 d1-3. Non-DAC group: all 47 patients were treated with standard 3+7 induction chemotherapy using cytarabine combined with idarubicin or mitoxantrone (IA for 41 cases, MA for 6 cases, the dose and usage are the same as before).
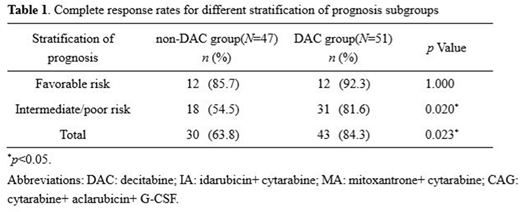
Results:In DAC group, the overall response rate after first course of induction therapy was 90.2%vs. 78.7% in non-DAC group, including 84.3% CR vs. 63.8% in non-DAC group (p=0.023). Curative effects in favorable prognosis group showed no pertinence to decitabine (85.7% vs. 92.3%, p=1.000). The decitabine combined therapy showed a favorable inductive remission rate of 81.6% in intermediate- or high-risk patients, while 54.5% in non-DAC group (p=0.020) (Table 1). Six patients were DNMT3A mutation positive in DAC group,. After the first course of chemotherapy, 4 reached CR (CR%=66.7). 16 patients were TET2 or ASXL1 mutation positive, with respective remission rate of 81.3% and 87.5% at the end of the first course. On hematologic toxicities, comparing with non-DAC group, the combined therapy did not extend the duration of agranulocytosis (p=0.0965) and platelet recovery time (p=0.1335). Relatively, DAC+CAG protocol had reduced the amount of infused suspension of red blood cells. Infection incidence showed no obvious difference between groups.
Conclusions:Decitabine combined chemotherapy could improve the inductive remission rate on MDS-like AML patients under 60 years old in the first course.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal